The effect of ProPhorce™ SR 130 on the control of necrotic enteritis
Despite being one of the main concerns in animal production, gut health is a complex issue that is difficult to define. Proper gut development and integrity, inflammation, and immunity, besides microbiome status are all elements involved in this concept. However, no single one on its own can draw the line between health and disease, or the impact on production. Each one of these elements is influenced by several stressors such as pathogens, toxins, nutrition, management, or the environment.
Challenge tests are valuable tools to estimate the impact of different stress factors on intestinal health and production. Necrotic enteritis is an infectious process with well-known and defined symptoms, which can replicate in experimental conditions. It is triggered by the joint action of two pathogens, Eimeria spp and C. perfringens, both of which can cause dysbiosis, inflammation, intestinal lesions, and mortality resulting in high economic losses. Necrotic enteritis challenge trials are a valuable model to evaluate the potential efficacy and usefulness of solutions for its control, but for other types of dysbiosis and more generic enteric processes as well.
In 2015, the Southern Poultry Research Group (SPRG) (USA) carried out a test on broilers following an established necrotic enteritis challenge model (Hofacre, 1998). The test aimed to evaluate the effect of ProPhorce™ SR 130 on production in challenged broilers and compare their results with the ones in birds supplemented with antibiotic grow promoters (Bacitracin-BMD). 4 treatment groups of birds were set and reared according to details described in table 1. The feeding program consisted of 3 phases: D1 to D21 (crumbs), D21 to D35 (pellets), D35 to D42 (pellets). Water and feed are provided ad libitum.
|
|
Challenge |
Treatment |
No. Birds |
|
T1 (Negative Control) |
NO |
NO |
600 (12 x 50) |
|
T2 (Positive Control) |
YES |
NO |
600 (12 x 50) |
|
T3 (ProPhorce™ SR 130) |
YES |
D1-D21: 500 gr/tn |
600 (12 x 50) |
|
T4 (Bacitracin-BMD) |
YES |
D1-D42: 50 gr/tn |
600 (12 x 50) |
2400 Ross x Ross chickens were selected and housed in 48 pens with 50 birds per pen. The 4 treatments were randomly distributed within the trial barn. All the birds were vaccinated against coccidiosis on day 0. On days 19-22, birds in groups T2, T3, and T4 were challenged with a toxigenic strain of C. perfringens. Production parameters were monitored (weight gain and conversion rate) on days 21, 35, and 42; evaluation of intestinal lesions was conducted on day 21 using the scoring criteria described in table 2.
|
Scoring of lesions |
Description |
|
T1 (Negative Control) |
Normal |
|
T2 (Positive Control) |
Light mucous layer covering the intestine |
|
T3 (ProPhorce™ SR 130) |
Small necrotic foci on the mucosa |
|
T4 (Bacitracin-BMD) |
Inflammation and haemorrhage in mucosa and intestinal contents |
The results (graph 3 and graph 4) show how the challenge had a significant impact on both feed conversion and weight gain. In this case, the Bacitracin group showed a limited effect on the losses in weight gain and conversion ratio caused by the challenge. Only the birds supplemented with ProPhorce™ SR 130 overcome the challenge and equals the performance of the negative (unchallenged) control group. These results are similar throughout the trial, both for the conversion ratio and average weight gain, giving consistency to the results.
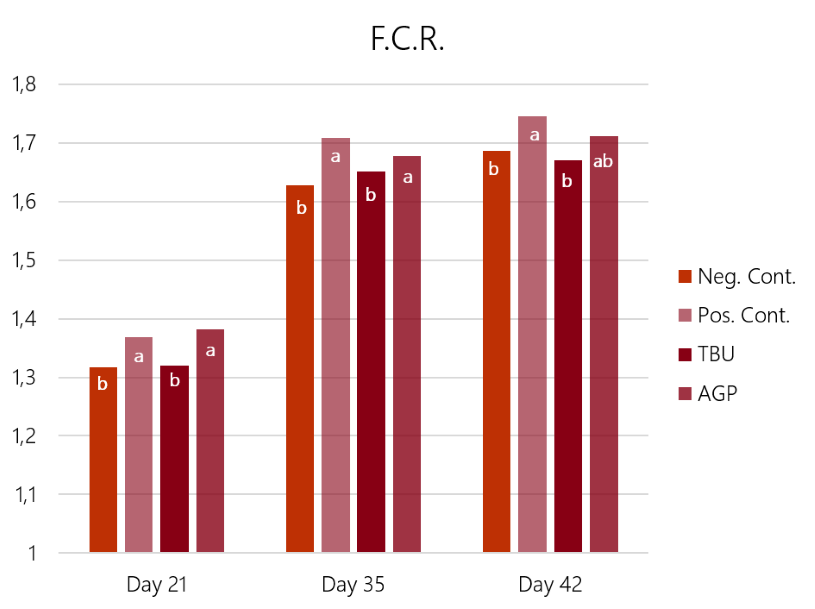 Graph 3: Feed conversion rate results of the 4 experimental groups, days 21, 32, and 42 of the trial. Different superscript letters (a, b) in the same set of bars show statistical differences (P<0.05)
Graph 3: Feed conversion rate results of the 4 experimental groups, days 21, 32, and 42 of the trial. Different superscript letters (a, b) in the same set of bars show statistical differences (P<0.05)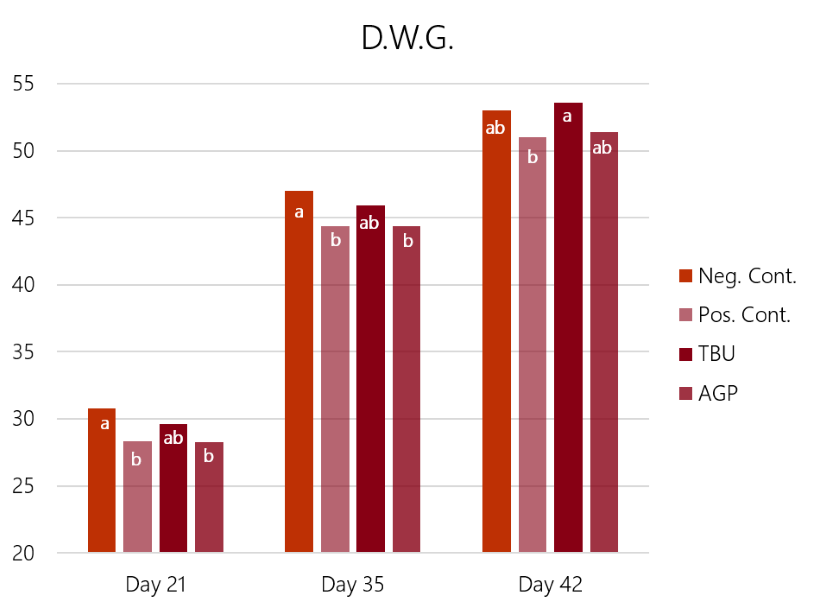 Graph 4: Average daily gain results of the 4 experimental groups, days 21, 32, and 42 of the trial. Different superscript letters (a, b) in the same set of bars show statistical differences (P<0.05)
Graph 4: Average daily gain results of the 4 experimental groups, days 21, 32, and 42 of the trial. Different superscript letters (a, b) in the same set of bars show statistical differences (P<0.05)
However, the results related to enteric lesion score (day 21) and mortality (day 42) show no significant differences between the challenged groups (table 5).
|
|
Lesion score |
Mortality |
|
T1 |
0.0a |
0.00a |
|
T2 |
0.3b |
6.86b |
|
T3 |
0.2b |
5.48b |
|
T4 |
0.3b |
5.14b |
These results are in line with previous experience that demonstrates the positive effect of tributyrin in intestinal mucosa protection from entero-pathogens, performance, and recovery after challenge.
As conclusions of this study, the birds that received ProPhorce™ SR 130 in their diets equaled the production results of the negative control group (non-challenged). These results might be explained by the capacity for protection and recovery of the intestinal mucosa by ProPhorce™ SR 130. There were no statistically significant differences between treated groups (T3 and T4), however, the results of ProPhorce™ SR 130 fed birds were numerically superior to those obtained with an antibiotic growth promoter in all controls points.
Butyric acid is recognized for its multiple effects related to intestinal health and integrity (Guilloteau, 2010); immune response modulation (Li, 2015); digestibility and absorption of nutrients (Peng, 2013); tissue repair (Alda, 2012); or the development of digestive flora and control of entero-pathogens (van der Wiellen, 2000). All these effects have a direct and positive impact on the maintenance and recovery of intestinal health besides the optimization of production results.
ProPhorce™ SR 130 (glycerol-ester of butyric acid) is a highly concentrated butyric acid source and the most efficient solution to deliver butyric acid into the intestine; these two traits together allow for low dosing and treatment cost.
The bibliography is available upon request.